Patient
portrayal

Learn more about CABOMETYX as a treatment for neuroendocrine tumors (NETs)* after prior treatment
*For pancreatic NET (pNET) or extra-pancreatic NET (epNET) that cannot be treated by surgery and have spread (locally advanced or metastatic).
What is CABOMETYX?
CABOMETYX is a kind of non-chemotherapy prescription medicine called a tyrosine kinase inhibitor (TKI). It is given as a tablet and taken once a day, or as directed.
It is used to treat:
- Adults and children 12 years of age and older with previously treated NETs that started inside or outside their pancreas.
How CABOMETYX may help
If you have NETs that are growing or spreading despite treatment, CABOMETYX could help.
In the clinical study, CABOMETYX was tested and evaluated in people after 1 or more NET treatments stopped working.
The CABOMETYX clinical study enrolled people with NETs that started inside or outside the pancreas. Their NETs had spread and progressed after 1 or more prior treatments (not including a somatostatin analog [SSA]). This clinical study was sponsored by the National Cancer Institute to meet the need for additional effective treatments for people with NETs whose condition is advancing.
A broad spectrum of people were evaluated in the clinical study. They had functional or nonfunctional NETs, all different grades, that had started in different locations:
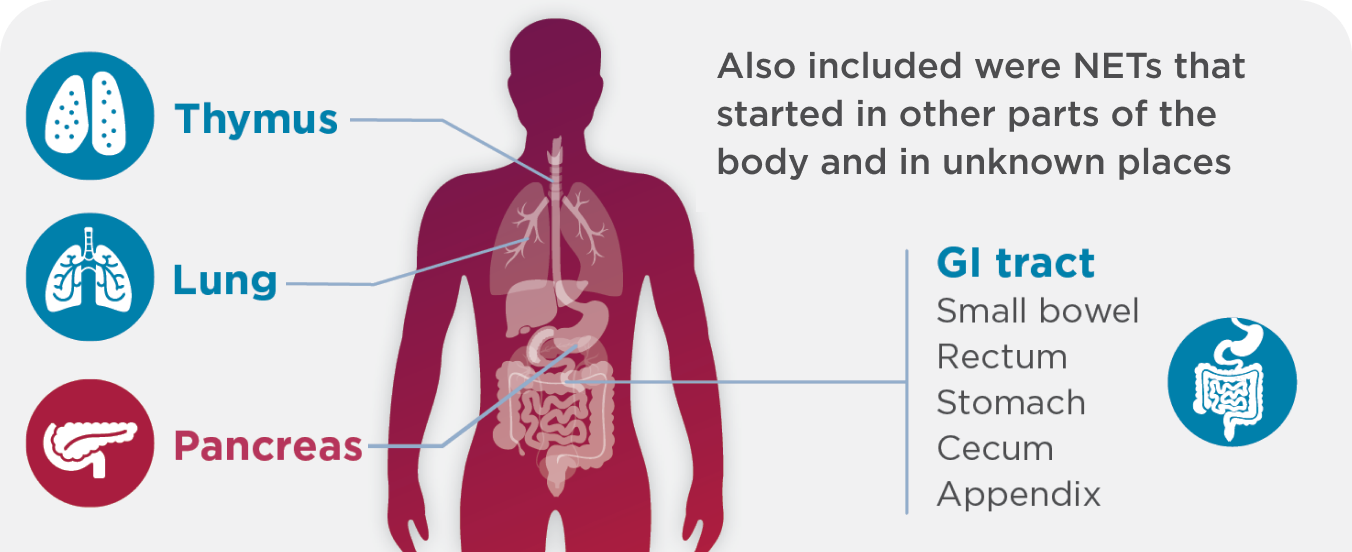
Some people in the study were taking an SSA, and all had previously received 1 or more treatments, which may have included a:

Peptide receptor radionuclide therapy (PRRT)

Targeted therapy

Chemotherapy
A total of 298 people participated in the study and were split into 2 groups:

99 people with NETs that started inside the pancreas

199 people with NETs that started anywhere outside the pancreas
Within these groups, people were randomly assigned to receive CABOMETYX or placebo (sugar pill). The study was blinded, which means that participants did not know what they received until after the study ended. This was done to prevent bias when reporting results.
The clinical study demonstrated a dramatic improvement in many of the people in the CABOMETYX group, so it was stopped early. The study then offered CABOMETYX to eligible people taking placebo.
People stayed on treatment until their tumor started to grow again, or they had a side effect that could not be managed.

This was the first Phase 3 study that included people who had prior PRRT for NETs that started inside or outside the pancreas.
Proven results of CABOMETYX in the clinical study
For NETs that started INSIDE the pancreas
On average, CABOMETYX helped people live longer without NETs growing or spreading
People were able to
LIVE WITHOUT
TUMOR GROWTH
4x
longer
Median* of 13.8 months for CABOMETYX vs 3.3 months for placebo
CABOMETYX reduced the risk of tumor growth or death by 78% vs placebo
Individual results may vary.
NETs in some people taking CABOMETYX shrunk,†
but not NETs in people taking placebo

CABOMETYX had a high tumor control rate
TUMORS STABILIZED‡
OR SHRUNK IN
80%
of people
In people taking CABOMETYX, 62% stabilized and 18% shrunk;
in people taking placebo, 55% stabilized and 0% shrunk
Please consider that these results are not definitive and individual results may vary.
At the time of this analysis, the results of CABOMETYX on overall survival were still being evaluated.
- *
-
Median is the middle value in a set of measurements—for some it was shorter; for others, longer.
- †
-
Tumors shrunk (partial response), but did not disappear.
- ‡
-
Stabilized means that changes in tumor sizes were too small to be reported as growing or shrinking
For NETs that started OUTSIDE the pancreas
On average, CABOMETYX helped people live longer without NETs growing or spreading
People were able to
LIVE WITHOUT
TUMOR GROWTH
2x
longer
Median* of 8.5 months for CABOMETYX vs 4.2 months for placebo
CABOMETYX reduced the risk of tumor growth or death by 60% vs placebo
Individual results may vary.
NETs in some people taking CABOMETYX shrunk,†
but not NETs in people taking placebo

CABOMETYX had a high tumor control rate
TUMORS STABILIZED‡
OR SHRUNK IN
69%
of people
In people taking CABOMETYX, 64% stabilized and 5% shrunk;
in people taking placebo, 52% stabilized and 0% shrunk
Please consider that these results are not definitive and individual results may vary.
At the time of this analysis, the results of CABOMETYX on overall survival were still being evaluated.
- *
-
Median is the middle value in a set of measurements—for some it was shorter; for others, longer.
- †
-
Tumors shrunk (partial response), but did not disappear.
- ‡
-
Stabilized means that changes in tumor sizes were too small to be reported as growing or shrinking.
Side Effects
Hear a Patient and Doctor Share Their Perspectives About Side Effects
Thomas Hutson, D.O., Pharm.D., and Rosa, an actual patient, talk about the importance of knowing what to expect with side effects of CABOMETYX.
This video features a real patient and doctor to show one individual’s treatment experience with CABOMETYX. Some participants were paid for their time and expenses in sharing their story. Individual results may vary. The information in this video is not intended as medical advice. Your healthcare team is your best resource for information about your treatment. If you have any questions about your condition or treatment, contact your healthcare team.
What are the most common side effects of CABOMETYX?
Taking CABOMETYX may cause side effects. The most common side effects of CABOMETYX include: tiredness, decreased appetite, nausea and vomiting, weight loss, and constipation.
Learn about additional side effects, including serious side effects associated with CABOMETYX here.
LEARN MORE ABOUT

Patient Handbook
Download this resource to learn more about CABOMETYX, and help you discuss with your doctor if it is right for you.

The BE CONNECTED support program provides educational information for you or someone you are caring for who is taking CABOMETYX.
